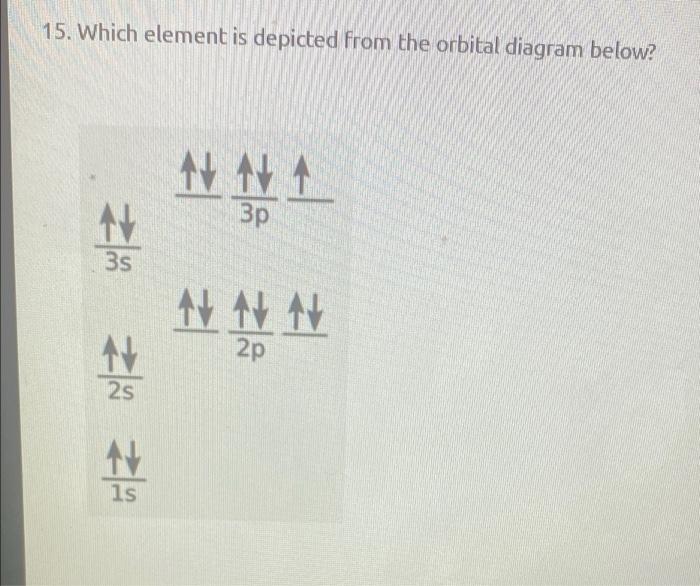
orbital diagram for neon
Neon is the tenth element with a total of 10 electrons. The electron configuration of iron ion Fe 3 is 1s 2 2s 2 2p 6 3s 2.

Neon Electron Configuration Ne With Orbital Diagram
In writing the electron configuration for neon the first two electrons will go in.
. Neon Orbital Diagram. 1s 2 2s 2 2p 6. He 2s 2 2p 6.
Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. The orbital diagram will be filled in the same order as described by the Aufbau principle. In the iodine ground-state electron configuration five.
Now To get the. The orbitals are p x p y and p z and each orbital. Electronic configuration of the Neon atom.
We already know that the p-subshell has three orbitals. What is the orbital diagram for iodine. Orbital diagram of Magnesium Mg 13.
Heres how you can draw the orbital diagram of neon step by step. The Basics of Orbital Diagrams. In writing the electron configuration for neon the.
The electron configuration of an atom is 1s 2 2s 2 2p 6. The iron atom donates two electrons in 4s orbital and an electron in 3d orbital to convert iron ion Fe 3. 1s 2s.
He 2s 2 2p 6. Neon Orbital Diagram Electron Configuration And Valence Electrons In the case of Neon we have the electron configuration is He 2s2 2p6 for this. Fill in the orbital energy diagram for the neon atom.
The full orbital diagram for neon is shown. Orbital diagram of Aluminum Al 14. 7 3 protons 4 number of neutrons in the atom.
Its also one of those chemical elements. Then to find the number of neutron round the atomic mass to the near whole number so atomic mass 6991 round to 7. Orbital diagrams Orbital box diagrams of all elements are mentioned in the.
Ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. Orbital diagram of Sodium Na 12. The number of electrons in the atom is.
Chemistry questions and answers. Electronic configuration of the Neon atom. So the electron configuration of.
2p El 2s 1s Submit Answer Try Another Version 2 item attempts remaining. 000084 gcm 3. Orbital diagram of Neon Ne 11.
The chemical element is also popular in chemistry due to its special tag as the second lightest gas after Helium. The first shell of Neon has 2 electrons and the outer shell or valence shell of Neon has 8 electrons hence the number of valence electrons in the Neon atom is 8. Fe 3e Fe 3.
The order in which the orbitals are filled with electrons from lower energy to higher energy is. Below is the electronic diagram. The ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3.
Reduced electronic configuration Ne. The above orbital diagram shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons and the 2p subshell has 6 electrons.

4 6 Electronic Configuration The Atom Siyavula

Orbital Filling Electron Configurations Where Do These Electrons Go Ppt Download
Image Electron Configuration Of Neon Atom

Electrons In Atoms Chapter 5 Chapter Big Idea The Atoms Of Each Element Have A Unique Arrangement Of Electrons Ppt Download
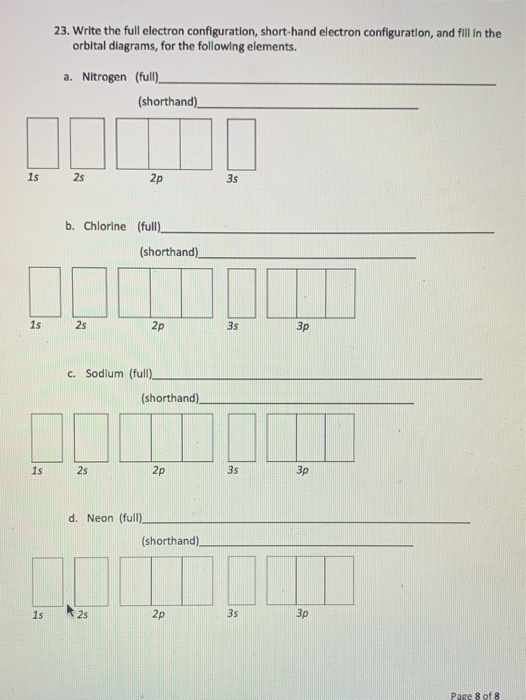
Solved 23 Write The Full Electron Configuration Short Hand Chegg Com
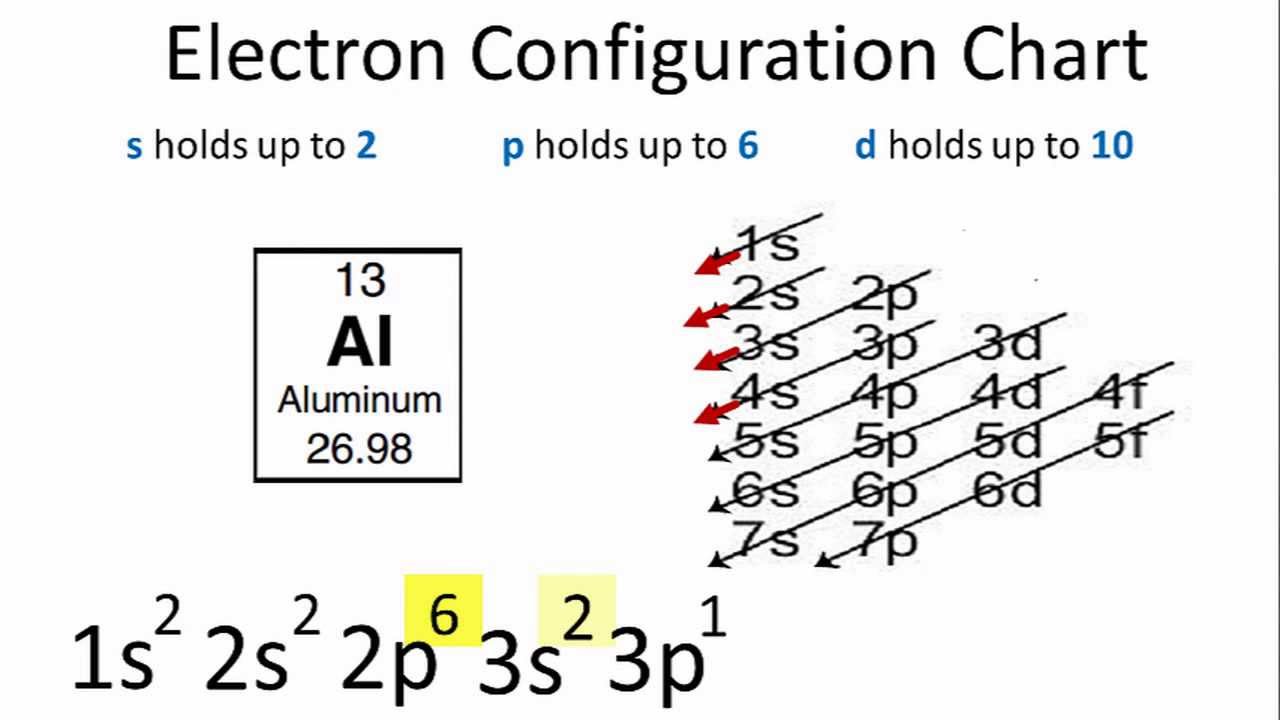
Electron Configuration For Aluminium Al
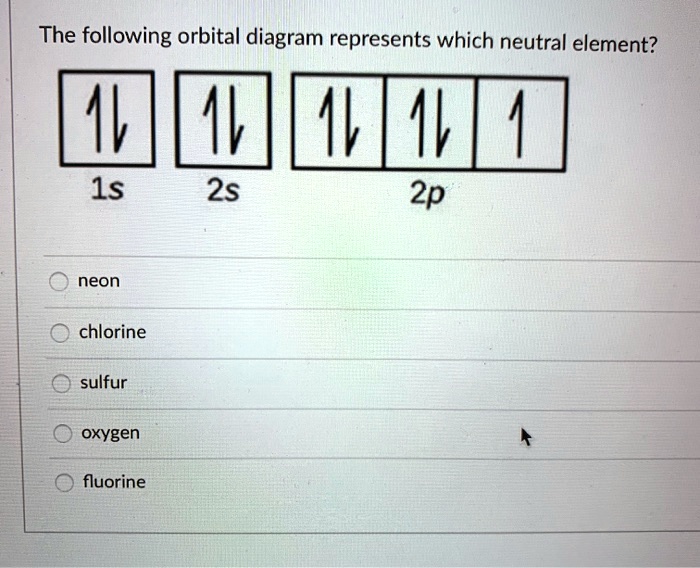
Solved The Following Orbital Diagram Represents Which Neutral Element 1v 1l 1 2s 2p 1s Neon Chlorine Sulfur Oxygen Fluorine

High School Chemistry Orbital Configurations Aufbau Principle High School Chemistry Chemistry

4 6 Electronic Configuration The Atom Siyavula

Solved Which Of The Following Orbital Diagrams Does Not Chegg Com

Electron Configurations And Orbital Diagrams Principles For Filling Orbitals Writing Electron Configurations Ppt Download

Orbital Diagrams And Electron Configuration Basic Introduction Chemistry Practice Problems Youtube

Neon Electron Configuration Electron Configuration Electrons Education

Molecular Orbital Of Ne2 Brainly In

Electron Configuration For Chlorine Cl
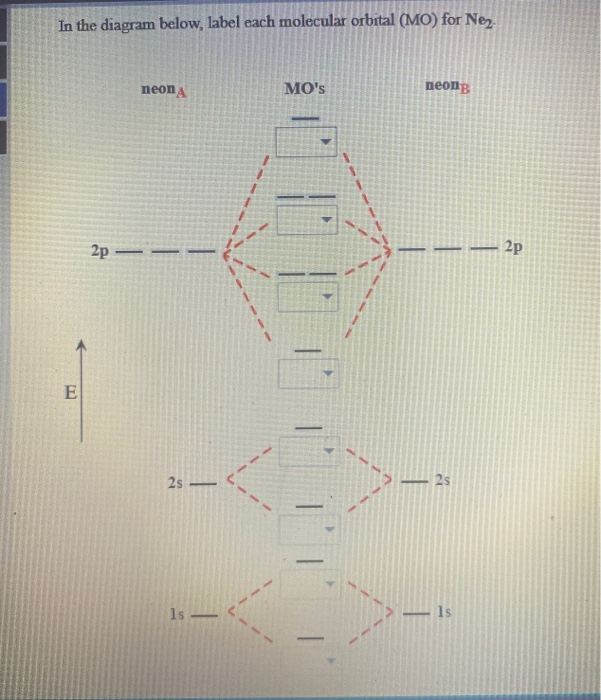
Solved In The Diagram Below Label Each Molecular Orbital Chegg Com

Electron Configuration Orbital Diagrams And Lewis Dot Test Alicia Barrett Library Formative